Saturday, November 27, 2010
Could be good
If he ends up writing about my work it will bring my mother great bragging points with her cousins whether he gets it right or not, but I would be greatly annoyed if he slaughters it.
Competing by not competing
Despite this, I am setting out to write a human demography paper, with little if any evolution in it. I can do this with some confidence because, as far as I know, I am working in an area that has been almost completely overlooked, and therefore I have no competition. Most anyone with a solid demography background could do a better job of what I want to do than I can, and I really couldn't compete. But because they haven't bothered, I don't have to compete. I can do a decent job and hopefully publish in a good journal without worrying about the competition, because I believe there to be none.
In my recent grant application, I wrote, "My work falls between demography and evolution, outside the well explored territory of either. Work within evolutionary demography tends to focus on senescence and reproduction; I have intentionally eschewed these to seek the question others have avoided. This is a high risk strategy; my work does not fit neatly into any one topic-specific journal or discipline. However this unconventional approach has the opportunity to found a new direction of study and investigate the most important questions therein." This is a polite way of saying that I intentionally avoid competition by seeking out the questions others have ignored, or deemed less interesting. Most successful scientists are successful because they look for opportunities to do what others aren't doing. I don't know to what extent other seek out whole areas that others haven't bothered with. I also don't know how successful or sustainable a strategy this is likely to be in the long run. After all, the success of the work will ultimately be measured by how many other people get interested in it, and try to improve upon it. Successful work, by definition, must therefore attract competition. So if I want to be successful, but continue not having competitors, I’ll need to move on to some other under-appreciated topic fairly quickly. As I rather like the topic I’m currently seeding, I may just have to put up with some competition, and try to stay ahead of them. having my own research group would be a great help in this. Not being an expert demographer is less of a problem if you have an expert demographer on staff.
Sunday, November 21, 2010
More Tardigrades
Anyway, here, by popular demand, are a couple more pictures.
Note the long curved claws at the ends of the toes, much like a bear has.
Saturday, November 20, 2010
Fussy Hydra babies
I find myself looking forward to having rotifers in the lab again. They are just so familiar at this point.
Friday, November 19, 2010
The Royal Society has informed me
Focus
Science, like most complex tasks, is much easier to make progress on if you know exactly what you are doing, and you have everything you need to do it ready. Over the last couple of months, I got a lot done because I focused on finishing my grant application, and on getting out the two papers I needed to finish to go with my application. Now the application is in, the review paper is accepted, and the methods paper is submitted, and I must find a new focus to guide me. Over the last couple of days I have been scattering my time and thoughts between a dozen projects, and not really making any progress on any of them. I am giving myself until Monday to decide what the new plan is.
Thursday, November 18, 2010
Tardigrades!
What is so cool about tardigrades? They can survive total dehydration, exposure to vacuum, radiation, freezing, extreme heat, conservative radio personalities, you name it. They can persist for a decade dried out with no signs of life, then just add water and they reanimate themselves. They are rolly and round and shaped kind of like six-legged teddy bears with two more legs growing out of their tails. This appearance has earned them the nickname ‘water bears.’
This afternoon I wanted to see if the hydra I am keeping in the lab would eat rotifers, because the Artemia I am giving them now are a bit too big for them, and rotifers a tiny. I found some in a puddle outside the institute, and in the same puddle, I found tardigrades. I had never knowingly seen a live tardigrade before, so I took a good long look at them under the microscope. These ones were well under a millimeter long, but I tried to get pictures.
They are small enough that really good photos would require a different microscope, but here are two.
This is a tardigrade from above. You can't really see how cute they are from above.
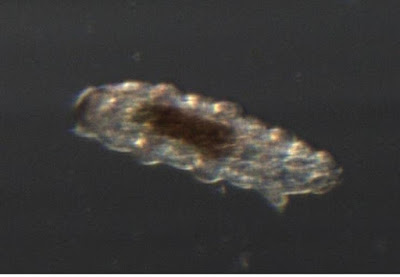
This is the shed skin of a tardigrade.
Just as snakes have to shed their stiff skins occasionally, many animals with exoskeletons molt that shell occasionally. All the arthropods (insects, spiders, crustaceans, crabs etc) will shed their skins, and so will tardigrades. Tardigrades are not arthropods, but are their closest realtive except for the velvet worms. This skin is head-end down. You can clearly see the six legs on the left side, and the two-legged tail at the top-left. I will try to get better pictures of a live critter tomorrow.
Sunday, November 14, 2010
Jon Asks: 2
In the Laura Ingalls Wilder book The Long Winter, Laura's father says that you can predict the severity of a winter by observing the thickness of muskrat nests in the summer. Muskrats, he says, will build thicker nests during the summer if the following winter is going to be relatively colder, and vice versa with thinner nests and relatively warmer winters. Has this folk wisdom been investigated? Is it true? And if so, where can I get my own muskrat colony?
I can't find anything on Google Scholar or Web of Science indicating that anyone has published anything about muskrats and weather prediction, other than this:
Man's natural craving for advance knowledge of coming weather extends thousands of years back of any attempts at scientific weather forecasting. Realizing that he has not the necessary foresight himself, he has imagined animals to be endowed with some peculiar sense which enables them to know, weeks or months ahead, what the weather will be. Thus a large group of animal weather proverbs has come into existence. Millions of people believe that the thickness of fur on a muskrat, or the number of nuts stored by a squirrel, or a supposedly early migration of certain birds, indicates a severe winter. Yet it is certain that animals have no such foresight.
from: Robert DeC. Ward. 1926. The Present Status of Long-Range Weather Forecasting
Proceedings of the American Philosophical Society,Vol. 65, No. 1, pp. 1-14
He provides no evidence to show that they don't, he is just certain. Note that the version you mention has to do with the thickness of the wall of the house, while the version Ward mentions has to do with the thicknesss of their fur. The fur hypothesis would be easier to test, if you were a muskrat hunter. I am frankly doubtful whether he or anyone else has done the work that would be needed to convincingly either story. You would have to measure the wall thickness of bunches of muskrat houses (or the pelt of many muskrats) in the summer. You would have to do this every year for quite a few years in order to make a convincing analysis of the relationship between wall thickness and hardness of winter. You would probably also want to measure various features of the microclimate, the muskrats behavior and physiology, and the local ecology, in order to get some sense of what the mechanism was. You probably would want to measure the pelts and the houses, just to make sure you were measuring the right thing. This is one limitation to testing folk-wisdom. There are often several versions, and it is hard to know if you are testing the right one unless you test all of them, and then you increase your chances of finding a strong correlation just by chance. My best guess is that there is some, but not a lot of, truth to either version of the story. Certainly they could pick up on whatever cues are available that the winter is going to be hard. But like most weather prediction, they probably aren't very accurate, at least not months in advance.
Jon Asks: 1
I've read that fungi are the only organisms that can degrade the longer-chain fibers in wood, such as lignin, and that without saprobic fungi the world would be blanketed in dead, undecayed trees. I see on Wikipedia that it is not literally true that no bacteria can degrade lignins, however, by Wiki's account, it does seem that no known bacteria are very good at it. (http://en.wikipedia.org/wiki/Ligninase) So why would that be the evolutionary case? Bacteria have evolved to break down pretty much everything else on the planet (roughly speaking), and wood has been around for something like 350+ million years. Why would they be such second-rate degraders when it comes to lignin?
This is an interesting question, but not one I can give a very satisfying answer to. Explanations of why something didn't evolve are always fairly speculative. Why no six legged tigers? Why no live-birthing birds? Why no Ents?
So why no lignin devouring bacteria? If they can do it poorly, why not well? Maybe it isn't worth their while to invest in that capacity, as they are always outcompeted by the fungi who can already do it? Maybe they can rely on the fungi to make the enzymes, and then they can just mooch. Perhaps the process of making the necessary enzymes requires separate cellular compartments, which bacteria lack. Maybe the necessary mutations just never occurred, and so couldn't be selected for. Certainly I don't know.
Friday, November 12, 2010
Winter
I have found that the best strategy for keeping my bad hip from minding the weather is to make sure I am absurdly over-dressed. This morning I walked to work, in temperatures above freezing, wearing high insulated shoes, long-underwear, lined pants, a heavy over-shirt, a down parka and thick gloves, hat and scarf. I was sweating the whole time, but my hip felt fine.
All of this does not endear the city to my heart, and yet I hope I will be here another five years. The grant application that is taking most of my time is to establish a research group of my own, funded by the Max Planck Society for five years. There are three responses they could make to my application. They could say no, they could say yes and let me form the research group here, or they could say yes but tell me to form it at a different Max Planck Institute, in a different city (i.e., Cologne).
I indicate in my application that my first choice is to stay here (at MPIDR), not out of love for the climate, but because of MPIDR’s, “strength in evolutionary demography would be a great benefit to me. I have ongoing collaborations with several MPIDR researchers, including members of the Research Groups on Lifecourse Dynamics and Demographic Change, and Modeling the Evolution of Aging, and of the Laboratories of Statistical Demography, Survival and Longevity, and Evolutionary Biodemography. As my project draws on evolutionary and demographic theory, on MPIDR's Human Mortality Database and Biodemographic Database, on laboratory experiments and survey data, MPIDR’s mixture of social science and biology is the ideal environment for me.”
Add to that that we have good friends, Iris enjoys teaching at the University here, we like our colleagues, we have a great apartment, and moving (which we have done far to much of) is a pain in the rear, and I am very much hoping to stay here. That said, if they offer me a group in Cologne, we will certainly go, in the spring.
Thursday, November 11, 2010
Good news
The point of a review article is to give an overview of the field. What is know, what is hypothesized, what are the important questions, where is the field going. I have been thinking about the topic of this one on and off for perhaps seven years, but if you put together all the time I specifically spent on it, it would be about six months of work, most in the last year. Perhaps half of that was spent just searching out the relevant literature. I must have read several thousand article titles, perhaps 500 abstracts, and maybe 200 full papers and book chapters. 91 sources made it into the final paper, and 18 more into the appendixes. The reason I had to do so much preliminary literature searching is that no one has ever written a review on this topic before, and perhaps four of the authors whose ideas and data I draw on had this general topic in mind.
Now, part of publishing with them and most other journals these days is that the paper is embargoed until they say it ain't. Embargoed means I can't tell the press what I found out, or even what the article is about, before the journal has a chance to publish it. But for the curious and bored, I can share the list of articles I reference. Just looking through them gives a sense of the range of journals I was searching in. Archiv fur Hydrobiologie, The American Journal of Tropical Medicine and Hygiene, Entomol. Exp. Appl, American Statistician, Obstetrical & Gynecological Survey, Can. J. Fish. Aquat. Sci., Experimental Gerontology, The Auk, Annals: New York Academy of Sciences, Biol. Reprod, Maturitas, Administrative Science Quarterly, J. Herpetol., Ophelia, Genetica, Journal of the Institute of Actuaries and so on. I am lucky in that I didn't have to actually scan the tables of contents of the several thousand journals that could potentially have had relevant papers. I used Google Scholar, Web of Science and other literature searching tools. I have no idea how they did this sort of thing before the internet.
Like a good playbill, in order of appearance:
Main Article:
1. Medawar, P. B. 1952 An unsolved problem of biology. London: HK Lewis.
2. Metcalf, C. J. E. & Pavard, S. 2007 Why evolutionary biologists should be demographers. Trends Ecol. Evol. 22, 205-212.
3. Wachter, K. W. 2008 Biodemography comes of age. Demographic Research 19, 1501-1512.
4. Vaupel, J. W. 2010 Biodemography of human ageing. Nature 464, 536-542.
5. Moorad, J. A. & Promislow, D. E. L. 2009 What can genetic variation tell us about the evolution of senescence? Proc. R. Soc. Lond., Ser. B: Biol. Sci.
6. Péron, G., Gimenez, O., Charmantier, A., Gaillard, J. M. & Crochet, P. A. 2010 Age at the onset of senescence in birds and mammals is predicted by early-life performance. Proceedings of the Royal Society B.
7. Young, H. 1963 Age-specific mortality in the eggs and nestlings of blackbirds. The Auk 80, 145-155.
8. Deevey, E. S. 1947 Life tables for natural populations of animals. The Quarterly Review of Biology 22, 283-314.
9. Jones, O. R., Gaillard, J. M., Tuljapurkar, S., Alho, J. S., Armitage, K. B., Becker, P. H., Bize, P., Brommer, J., Charmantier, A. & Charpentier, M. 2008 Senescence rates are determined by ranking on the fast–slow life-history continuum. Ecol. Lett. 11, 664-673.
10. Pike, D. A., Pizzatto, L., Pike, B. A. & Shine, R. 2008 Estimating survival rates of uncatchable animals: The myth of high juvenile mortality in reptiles. Ecology 89, 607-611.
11. Carey, J. R., Liedo, P. & Vaupel, J. W. 1995 Mortality dynamics of density in the Mediterranean fruit fly. Experimental Gerontology 30, 605-629.
12. Pletcher, S. D., Macdonald, S. J., Marguerie, R., Certa, U., Stearns, S. C., Goldstein, D. B. & Partridge, L. 2002 Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr. Biol. 12, 712-723.
13. Rose, M. R. 1984 Laboratory evolution of postponed senescence in Drosophila melanogaster. Evolution 38, 1004-1010.
14. Tatar, M., Carey, J. R. & Vaupel, J. W. 1993 Long-term cost of reproduction with and without accelerated senescence in Callosobruchus maculatus: analysis of age-specific mortality. Evolution 47, 1302-1312.
15. Bauer, G. 1983 Age structure, age specific mortality rates and population trend of the freshwater pearl mussel(Margaritifera margaritifera) in North Bavaria. Archiv fur Hydrobiologie 98, 523-532.
16. Styer, L. M., Carey, J. R., Wang, J. L. & Scott, T. W. 2007 Mosquitoes do senesce: departure from the paradigm of constant mortality. The American Journal of Tropical Medicine and Hygiene 76, 111.
17. Sarup, P. & Loeschcke, V. 2010 Life extension and the position of the hormetic zone depends on sex and genetic background in Drosophila melanogaster. Biogerontology, 1-9.
18. Caughley, G. 1966 Mortality patterns in mammals. Ecology 47, 906-918.
19. Barlow, J. & Boveng, P. 1991 Modeling age-specific mortality for marine mammal populations. Mar. Mamm. Sci. 7, 50-65.
20. Spinage, C. A. 1972 African ungulate life tables. Ecology 53, 645-652.
21. Bruderl, J. & Schussler, R. 1990 Organizational mortality: The liabilities of newness and adolescence. Administrative Science Quarterly 35, 530-547.
22. Yang, G. 2007 Life cycle reliability engineering. Hoboken, NJ: John Wiley & Sons Inc.
23. Holliday, R. 2006 Aging is no longer an unsolved problem in biology. Annals: New York Academy of Sciences 1067, 1-9.
24. Hamilton, W. D. 1966 The moulding of senescence by natural selection. J. Theor. Biol. 12, 12-45.
25. Williams, G. C. 1957 Pleiotropy, Natural Selection, and the Evolution of Senescence. Evolution 11, 398-411.
26. Baudisch, A. 2008 Inevitable aging?: contributions to evolutionary-demographic theory: Springer Verlag.
27. Kirkwood, T. B. L. 1990 The disposable soma theory of aging. In Genetic effects on aging (ed. D. E. Harrison), pp. 9–19. Caldwell, NJ: Telford Press.
28. Holman, D. J. & Wood, J. W. 2001 Pregnancy loss and fecundability in women. In Reproductive ecology and human evolution (ed. P. T. Ellison), pp. 15–38. Hawthorne, NY: Aldine de Gruyter.
29. Woods, R. 2009 Death before Birth. Oxford: Oxford University Press.
30. O'Connor, K. A., Holman, D. J. & Wood, J. W. 1998 Declining fecundity and ovarian ageing in natural fertility populations. Maturitas 30, 127-136.
31. Kruger, D. J. & Nesse, R. M. 2004 Sexual selection and the Male:Female Mortality Ratio. Evolutionary Psychology 2, 66-85.
32. Bonduriansky, R. & Brassil, C. E. 2002 Senescence: rapid and costly ageing in wild male flies. Nature 420, 377.
33. McDonald, D. B., Fitzpatrick, J. W. & Woolfenden, G. E. 1996 Actuarial senescence and demographic heterogeneity in the Florida Scrub Jay. Ecology 77, 2373-2381.
34. Roach, D. A., Ridley, C. E. & Dudycha, J. L. 2009 Longitudinal analysis of Plantago: age-by-environment interactions reveal aging. Ecology 90, 1427.
35. Nussey, D. H., Coulson, T., Festa-Bianchet, M. & Gaillard, J. M. 2008 Measuring senescence in wild animal populations: towards a longitudinal approach. Funct. Ecol. 22, 393-406.
36. Sheader, M. 2009 The reproductive biology and ecology of Gammarus duebeni (Crustacea: Amphipoda) in southern England. Journal of the Marine Biological Association of the UK 63, 517-540.
37. Milnes, M. R., Bryan, T. A., Katsu, Y., Kohno, S., Moore, B. C., Iguchi, T. & Guillette, L. J. 2008 Increased posthatching mortality and loss of sexually dimorphic gene expression in alligators (Alligator mississippiensis) from a contaminated environment. Biol. Reprod. 78, 932.
38. Anderson, D. J. 1990 Evolution of obligate siblicide in boobies. 1. A test of the insurance-egg hypothesis. Am. Nat. 135, 334-350.
39. Strandberg, R., Klaassen, R. H. G., Hake, M. & Alerstam, T. 2009 How hazardous is the Sahara Desert crossing for migratory birds? Indications from satellite tracking of raptors. Biol. Lett.
40. Moss, C. J. 2006 The demography of an African elephant (Loxodonta africana) population in Amboseli, Kenya. J. Zool. 255, 145-156.
41. Sumich, J. L. & Harvey, J. T. 1986 Juvenile mortality in gray whales (Eschrichtius robustus). J. Mammal. 67, 179-182.
42. Anderson, J. T. 1988 A review of size dependent survival during pre-recruit stages of fishes in relation to recruitment. Journal of Northwest Atlantic Fisheries Science 8, 55-66.
43. Gislason, H., Daan, N., Rice, J. C. & Pope, J. G. 2010 Size, growth, temperature and the natural mortality of marine fish. Fish Fish. 11, 149-158.
44. Baron, J. P., Le Galliard, J. F., Tully, T. & Ferrière, R. 2010 Cohort variation in offspring growth and survival: prenatal and postnatal factors in a late-maturing viviparous snake. J. Anim. Ecol. 79, 640-649.
45. Kushlan, J. A. & Jacobsen, T. 1990 Environmental variability and the reproductive success of Everglades alligators. J. Herpetol. 24, 176-184.
46. Congdon, J. D., Dunham, A. E. & Van Loben, R. C. 1993 Delayed sexual maturity and demographics of Blanding's turtles (Emydoidea blandingii): implications for conservation and management of long-lived organisms. Conserv. Biol. 7, 826-833.
47. Petranka, J. W. 1985 Does age-specific mortality decrease with age in amphibian larvae? Copeia 1985, 1080-1083.
48. Wilbur, H. M. 1980 Complex life cycles. Annu. Rev. Ecol. Syst. 11, 67-93.
49. Miller, T. J., Crowder, L. B., Rice, J. A. & Marschall, E. A. 1988 Larval size and recruitment mechanisms in fishes: toward a conceptual framework. Can. J. Fish. Aquat. Sci. 45, 1657-1670.
50. Rabinovich, J. E., Nieves, E. L. & Chaves, L. F. 2010 Age-specific mortality analysis of the dry forest kissing bug, Rhodnius neglectus. Entomol. Exp. Appl. 135, 252-262.
51. Chapman, A. R. O. 1986 Age versus stage: An analysis of age-and size-specific mortality and reproduction in a population of Laminaria longicruris Pyl. J. Exp. Mar. Biol. Ecol. 97, 113-122.
52. Cole, K. M. & Sheath, R. G. 1990 Biology of the red algae: Cambridge Univ Pr.
53. Hett, J. M. 1971 A dynamic analysis of age in sugar maple seedlings. Ecology 52, 1071-1074.
54. Leak, W. B. 1975 Age distribution in virgin red spruce and northern hardwoods. Ecology 56, 1451-1454.
55. Harcombe, P. A. 1987 Tree life tables. Bioscience 37, 557-568.
56. Leverich, W. J. & Levin, D. A. 1979 Age-specific survivorship and reproduction in Phlox drummondii. Am. Nat. 113, 881-903.
57. Hanley, M. E., Fenner, M. & Edwards, P. J. 1995 The effect of seedling age on the likelihood of herbivory by the slug Deroceras reticulatum. Funct. Ecol. 9, 754-759.
58. Gosselin, L. A. & Qian, P. Y. 1997 Juvenile mortality in benthic marine invertebrates. Mar. Ecol. Prog. Ser. 146, 265-282.
59. Hallock, P. 1985 Why are larger foraminifera large? Paleobiology 11, 195-208.
60. Levin, L., Caswell, H., Bridges, T., DiBacco, C., Cabrera, D. & Plaia, G. 1996 Demographic responses of estuarine polychaetes to pollutants: life table response experiments. Ecol. Appl. 6, 1295-1313.
61. Greeff, J. M., Storhas, M. G. & Michiels, N. K. 1999 Reducing losses to offspring mortality by redistributing resources. Funct. Ecol., 786-792.
62. Klug, H. & Bonsall, M. B. 2007 When to care for, abandon, or eat your offspring: the evolution of parental care and filial cannibalism. The American Naturalist 170, 886.
63. Manica, A. 2002 Filial cannibalism in teleost fish. Biological Reviews 77, 261-277.
64. Lee, R. D. 2003 Rethinking the evolutionary theory of aging: Transfers, not births, shape senescence in social species. Proc. Natl. Acad. Sci. USA 100, 9637-9642.
65. Rogers, A. R. 2003 Economics and the evolution of life histories. Proc. Natl. Acad. Sci. USA 100, 9114-9115.
66. Lee, R. 2008 Sociality, selection, and survival: Simulated evolution of mortality with intergenerational transfers and food sharing. Proc. Natl. Acad. Sci. USA 105, 7124-7128.
67. Rumrill, S. S. 1990 Natural mortality of marine invertebrate larvae. Ophelia 32.
68. Jones, D. & Göth, A. 2008 Mound-builders. Collingwood VIC: CSIRO.
69. Chu, C. Y. C., Chien, H. K. & Lee, R. D. 2007 Explaining the optimality of U-shaped age-specific mortality. Theor. Popul. Biol. 73, 171-180.
70. Biro, P. A., Abrahams, M. V., Post, J. R. & Parkinson, E. A. 2006 Behavioural trade-offs between growth and mortality explain evolution of submaximal growth rates. Ecology 75, 1165-1171.
71. Sterck, F. J., Poorter, L. & Schieving, F. 2006 Leaf traits determine the growth-survival trade-off across rain forest tree species. Am. Nat. 167, 758-765.
72. Soler, J. J., de Neve, L., Pérez-Contreras, T., Soler, M. & Sorci, G. 2003 Trade-off between immunocompetence and growth in magpies: an experimental study. Proc. R. Soc. Lond., Ser. B: Biol. Sci. 270, 241-248.
73. Mangel, M. & Stamps, J. 2001 Trade-offs between growth and mortality and the maintenance of individual variation in growth. Evol. Ecol. Res. 3, 583-593.
74. Johnson, D. W. & Hixon, M. A. 2010 Ontogenetic and spatial variation in size-selective mortality of a marine fish. J. Evol. Biol. 23, 724-737.
75. Munch, S. B. & Mangel, M. 2006 Evaluation of mortality trajectories in evolutionary biodemography. Proc. Natl. Acad. Sci. USA 103, 16604-16607.
76. von Bertalanffy, L. 1957 Quantitative laws in metabolism and growth. The Quarterly Review of Biology 32, 217-231.
77. Vaupel, J. W., Manton, K. G. & Stallard, E. 1979 The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography 16, 439-454.
78. Vaupel, J. W. & Yashin, A. I. 1985 Heterogeneity's ruses: some surprising effects of selection on population dynamics. American Statistician 39, 176-185.
79. Charlesworth, B. 2001 Patterns of age-specific means and genetic variances of mortality rates predicted by the mutation-accumulation theory of ageing. J. Theor. Biol. 210, 47-65.
80. Mueller, L. D. & Rose, M. R. 1996 Evolutionary theory predicts late-life mortality plateaus. Proc. Natl. Acad. Sci. USA 93, 15249-15253.
81. Moorad, J. A. & Promislow, D. E. L. 2008 A theory of age-dependent mutation and senescence. Genetics 179, 2061-2073.
82. Gong, Y., Thompson Jr, J. N. & Woodruff, R. C. 2006 Effect of deleterious mutations on life span in Drosophila melanogaster. Journals of Gerontology Series A: Biological and Medical Sciences 61, 1246-1252.
83. Pletcher, S. D., Houle, D. & Curtsinger, J. W. 1998 Age-specific properties of spontaneous mutations affecting mortality in Drosophila melanogaster. Genetics 148, 287-303.
84. Yampolsky, L. Y., Pearse, L. E. & Promislow, D. E. L. 2000 Age-specific effects of novel mutations in Drosophila melanogaster I. Mortality. Genetica 110, 11-29.
85. Wexler, N. S. 2004 Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington's disease age of onset. Proc. Natl. Acad. Sci. USA 101, 3498-3503.
86. Wright, S. 1929 Fisher's theory of dominance. Am. Nat. 63, 274-279.
87. Orr, H. A. 1991 A test of Fisher's theory of dominance. Proc. Natl. Acad. Sci. USA 88, 11413-11415.
88. Arbeitman, M. N., Furlong, E. E. M., Imam, F., Johnson, E., Null, B. H., Baker, B. S., Krasnow, M. A., Scott, M. P., Davis, R. W. & White, K. P. 2002 Gene expression during the life cycle of Drosophila melanogaster. Science 297, 2270.
89. Martinez, D. E. 1998 Mortality patterns suggest lack of senescence in hydra. Experimental Gerontology 33, 217-225.
90. Bakketeig, L. S., Seigel, D. G. & Sternthal, P. M. 1978 A fetal-infant life table based on single births in Norway, 1967-1973. Am. J. Epidemiol. 107, 216.
91. Human Life-Table Database. 2010 www.lifetable.de: Max Planck Institute for Demographic Research, Institut national d'études démographiques and UC Berkeley
Appendix 1.
1. Bourgeois-Pichat, J. 1946 De la mesure de la mortalité infantile. Population 1, 53-68.
2. Bourgeois-Pichat, J. 1951 La mesure de la mortalité infantile. II. Les causes de décès. Population 6, 459-480.
3. Wilmoth, J. R. 1997 In search of limits. In Between Zeus and the salmon: The biodemography of longevity (ed. K. W. Wachter), pp. 38-64. Washington, DC: National Academies Press.
4. Carnes, B. A., Holden, L. R., Olshansky, S. J., Witten, M. T. & Siegel, J. S. 2006 Mortality partitions and their relevance to research on senescence. Biogerontology 7, 183-198.
5. Siler, W. 1979 A competing-risk model for animal mortality. Ecology 60, 750-757.
6. Gage, T. B. 1998 The comparative demography of primates: with some comments on the evolution of life histories. Annual Review of Anthropology 27, 197-221.
7. Heligman, L. & Pollard, J. H. 1980 The age pattern of mortality. Journal of the Institute of Actuaries 107, 49–80.
8. Lee, E. T. & Go, O. T. 1997 Survival analysis in public health research. Annu. Rev. Public Health 18, 105-134.
9. Ndirangu, J., Newell, M. L., Tanser, F., Herbst, A. J. & Bland, R. 2010 Decline in early life mortality in a high HIV prevalence rural area of South Africa: evidence of HIV prevention or treatment impact? AIDS 24, 593.
Appendix 2
1. Bishop, M. W. H. 1964 Paternal contribution to embryonic death. Reproduction 7, 383-396.
2. Wilcox, A. J., Weinberg, C. R., O'Connor, J. F., Baird, D. D., Schlatterer, J. P., Canfield, R. E., Armstrong, E. G. & Nisula, B. C. 1989 Incidence of early loss of pregnancy. Obstetrical & Gynecological Survey 44, 147.
3. Boklage, C. E. 1990 Survival probability of human conceptions from fertilization to term. International Journal of Fertility 35, 75-94.
4. O'Connor, K. A., Holman, D. J. & Wood, J. W. 1998 Declining fecundity and ovarian ageing in natural fertility populations. Maturitas 30, 127-136.
5. Hassold, T. & Hunt, P. 2001 To err (meiotically) is human: the genesis of human aneuploidy. Nature Reviews Genetics 2, 280-291.
6. Beatty, R. A. 2008 The genetics of the mammalian gamete. Biological Reviews 45, 73-117.
7. Wilmut, I., Sales, D. I. & Ashworth, C. J. 1986 Maternal and embryonic factors associated with prenatal loss in mammals. Reproduction 76, 851-864.
8. Bloom, S. E. 1972 Chromosome abnormalities in chicken (Gallus domesticus) embryos: types, frequencies and phenotypic effects. Chromosoma 37, 309-326.
9. Forstmeier, W. & Ellegren, H. 2010 Trisomy and triploidy are sources of embryo mortality in the zebra finch. Proc. R. Soc. Lond., Ser. B: Biol. Sci.
Monday, November 08, 2010
A botanist once told me...
Botanists have to make up dirty rhymes because they can't just take fecal samples the way zoologists do.
Monday, November 01, 2010
Halloween, diversity, and the left-leaning scientists
One could easily suggest several possible reasons for left-lean. The liberal might say that scientists are people trained to think carefully about ideas, and liberal ideas stand up to careful thought better than their conservative counterparts. She could add that conservatives, particularly the United States, tend to engage in a great deal of anti-intellectualism, which is not a good way to win the support of scientists and that conservative leaders and movements twist scientific conclusions and ideas more frequently and more disastrously than do liberals. A conservative could argue that as a big-spending liberals tend to support science funding, scientists have a direct interest in supporting liberals. I think all of these things are at least sometimes true.
Another hypothesis occurred to me last night during our Halloween party. Looking around our apartment, I saw not only several Americans (tiger, mime, fisherman, pirate, little girl/trick-or-treater, scarecrow, frog, Dorothy, lumberjack, but no tricorn hats) and Germans (lizard, clown, flapper, schoolgirl, bus stop, Little-Red-Riding-Hood, ninja, and a little boy dressed as a little boy), but also friends from Italy (a mouse and a mummy), Hungary (bank robber), Austria (ghost and witch), Australia (tin man and geisha), Poland (burglar, witches and a ghost), Latvia (witch), Spain (ghost and sorceress) and Finland (clown, penguin, bear and ladybug). We had 34 people from 10 countries, and had invited people from at least a dozen more (Japan, Taiwan, China, Mexico, Brazil, the Netherlands, Denmark, England, Russia, Bulgaria, Turkey and New Zealand). This sort of national diversity is common at gatherings of Institute researchers. I had a similar experience at Berkeley, with colleagues from all over the world.
Conservatives in many countries are nationalistic, or at least view the traditional ways of doing things in their part of the world as best and most correct. So perhaps the conservative hoping to build a career in science finds herself in many uncomfortably international situations, where her assumptions are challenged or potententally unpopular. If so, such people may tend to either revise their opinions (or pretend to, which often eventually leads to an actual change) or avoid such situations. Such avoidance would make a successful career in science difficult, at least at the prestigious institutions which tend to have international research staff.
The Ivory Tower's relative lack of political diversity may partly result from its high demographic diversity. It is hard to condemn witches when your living room is full of them.



